uv spectroscopy transitions|Ultraviolet and Visible Spectroscopy : Baguio Objectives. discuss the bonding in 1,3-butadiene in terms of the molecular orbital theory, and draw a molecular orbital for this and similar compounds. understand how electronic transitions occur. get an . The official STL result today, April 23, 2024 (Tuesday) Visayas, Mindanao is available here at 10:30AM, 3PM, 5PM, 7PM, 8PM and 9PM. . 2023 following the directive on the use of national games draw results for STL. . Laguna bettor wins Php 30.2-M Mega Lotto jackpot . August 6, .
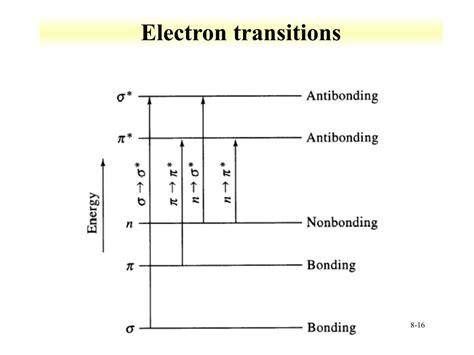
uv spectroscopy transitions,Of the six transitions outlined, only the two lowest energy ones, n to pi* and pi to pi* (colored blue) are achieved by the energies available in the .
In this chapter, we have briefly described the UV-VIS spectroscopy by covering the fundamentals of UV-VIS spectroscopy, origin of spectra along with the types of electronic . Ultraviolet-Visible (UV-Vis) spectroscopy is a versatile and powerful analytical method, which allows to investigate a wide variety of catalysts in both the liquid-phase and . Objectives. discuss the bonding in 1,3-butadiene in terms of the molecular orbital theory, and draw a molecular orbital for this and similar compounds. understand how electronic transitions occur. get an . Where UV-vis spectroscopy becomes useful to most organic and biological chemists is in the study of molecules with conjugated pi systems. In these groups, the energy gap for π - π * transitions is smaller than for .Transition metal compounds are coloured because the energy involved with these transitions occurs in the visible part of the spectrum. Click the image for an interactive Flash animation enriching this concept.Most absorption spectroscopy of organic compounds is based on transitions of n or p electrons to the p * excited state. This is because the absorption peaks for these transitions fall in an .UV-Visible Spectroscopy. Visible and Ultraviolet Spectroscopy. 1. Background. An obvious difference between certain compounds is their color.

with the precise amount of energy can cause transitions from one level to another will be absorbed. The possible electronic transitions that light might cause are2: . UlTRAVIOlET - VISIBlE SPEcTROScOPY (UV) INTROdUcTION 4 Maximum absorption at this wavelength absorbance 1.0 0.8 0.6 0.4 0 200 220 240 260 280 300 wavelength (nm) max=217nm
While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in the UV (200-400 nm) and visible (400-700 nm) range of the electromagnetic .
Where UV-vis spectroscopy becomes useful to most organic and biological chemists is in the study of molecules with conjugated pi systems. In these groups, the energy gap for π - π * transitions is smaller than for .
While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in the UV (200-400 nm) and visible (400-700 nm) range of the electromagnetic spectrum causes many organic molecules to undergo electronic transitions.What this means is that when the energy from UV or visible light is . While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in the UV (200-400 nm) and visible (400-700 nm) range of the electromagnetic spectrum causes many organic molecules to undergo electronic transitions.What this means is that when the energy from UV or visible light is .

In UV-Vis spectroscopy, wavelength is usually expressed in nanometers (1 nm = 10-9 m). It follows from the equations that radiation with shorter wavelength has higher energy, and, for UV-Vis spectroscopy, the low (short) Figure 2. Electronic transitions in formaldehyde. UV light at 187 nm causes excitation ofUV and visible absorption of transition metal complexes. Ultraviolet and visible absorption spectroscopy involve transitions between electron energy levels in atoms and molecules where the energy difference corresponds to the ultraviolet and visible regions of the electromagnetic spectrum. Wales. A/AS level. WJEC Chemistry
TRANSITIONS IN ULTRAVIOLET SPECTROSCCOPY Electronic transitions in UV-visible spectroscopy which are important are n→ π * & π→ π * transitions. (a) n→ π * transitions. - In this transition, an electon of unshared electron pair on a hetero atom is excited to π * antibonding orbital. This transition involves least amount of energy than .
Khan Academy Great discussion of the topic!! Question on Azo yellow dye. When I look up the absorption spectrum of cis azo yellow on the internet, I see that the absorption peak at ~420 is much weaker than the peak further into the UV, which would be expected for an n->pi star transition for the reasons you explained, but the lambda max for the stronger shorter . UV Spectroscopy involves the promotion of electrons (n, σ, π) from the ground state to a higher energy state. UV Spectroscopy Parts and Uses. . Types of Electronic Transitions. According to the molecular orbital .
The UV-Vis spectrometer is a useful tool because it allows us to nail down exactly where samples absorb light, and thus quantify electronic transitions. For example, knowing that the λ max for ethene is at 174 nm .The ultraviolet region falls in the range between 190-380 nm, the visible region fall between 380-750 nm. The following electronic transitions are possible: π-π * (pi to pi star transition) n-π * (n to pi star transition) σ - σ * (sigma to sigma .uv spectroscopy transitions Ultraviolet spectroscopy provides much less information about the structure of molecules than do the spectroscopic techniques studied earlier (infrared spectroscopy, mass spectroscopy, and NMR spectroscopy). . Of the six transitions outlined, only the two lowest energy ones (left-most, colored blue) are achieved by the energies available in .5. Ultraviolet–Visible Spectroscopy UV-Visible spectroscopy deals with the study of the electronic transitions of molecules as they absorb light in the UV (190-400 nm) and visible regions (400-800 nm) of the electromagnetic spectrum. The absorption of ultraviolet or visible radiation lead to transition among electronic UV-Vis Spectroscopy or Ultraviolet-visible spectroscopy or Ultraviolet-visible spectrophotometer (UV-Vis) is also called absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. . A smoother transition is possible when the switchover occurs between 300 and 350 nm because the light emission for both .
These electronic transitions interact with photons very efficiently. As a result, there is a kind of counterintuitive relationship in the UV-visible spectra of transition metal complexes: d-d transitions require very little energy but occur relatively infrequently, meaning they give very weak absorbances in the spectrum.uv spectroscopy transitions Ultraviolet and Visible Spectroscopy Of the six transitions outlined, only the two lowest energy ones (left-most, colored blue) are achieved by the energies available in the 200 to 800 nm spectrum. As a rule, . The presence of chromophores in a molecule is best documented by UV-Visible spectroscopy, but the failure of most instruments to provide absorption data for wavelengths .Ultraviolet-visible (UV-Vis) spectroscopy is one of the most popular analytical techniques because it is very versatile and able to detect nearly every molecule. With UV-Vis spectroscopy, the UV-Vis light is passed through a sample and the transmittance of light by a sample is measured. . The most common transitions that fall in the UV-Vis .
uv spectroscopy transitions|Ultraviolet and Visible Spectroscopy
PH0 · Ultraviolet–visible (UV
PH1 · Ultraviolet and visible spectroscopy
PH2 · Ultraviolet and Visible Spectroscopy
PH3 · Ultraviolet
PH4 · Ultraviolet
PH5 · UV
PH6 · The Basics of UV
PH7 · 3.3: Electronic Transitions